In this campaign year, politicians have once again started to consider the issue of regulating pharmaceutical pricing policies. It’s not a new concept but recent practices of certain pharmaceutical companies have given this topic new life.
An article in The New York Times on October 4th reported on several pharmaceutical companies that were buying existing patented therapeutic drugs and dramatically increasing the price of the drug to increase revenue and profitability (see the article here). In some cases the drug prices increase hundreds of percent overnight, the article reported. The article also pointed out that many consumers are unable to meet these new costs or fear that their current health insurance plan will stop covering these necessary medications. The article pointed out that these companies, which have very high profit margins and growth rates typically spent less than 5% of their revenue on Research and Development, while most pharmaceutical companies spent 15-20% of their revenue developing new drugs. A representative from one of the companies in question defended the company’s price practices and indicated that it “prices its treatments based on a range of factors, including clinical benefits and the value they bring to patients, physicians, payers and society.”
We wanted to know what scientist thought about the idea of regulating pharmaceutical pricing practices. We polled a total of 2,191 US scientists (evenly divided between industrial and non-profit laboratories) regarding their opinions on this issue. After summarizing The New York Times article mentioned above and providing a link so they could read it themselves we asked the following question:
“In your opinion, should these pricing practices for existing drug sales in the US be regulated by the United States federal government?”
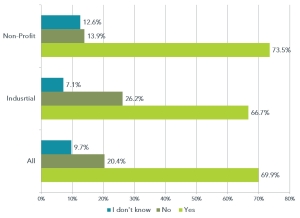
We received 319 total responses (168 industrial and 158 non-profit participants). The results were interesting (see figure).
Industrial scientist (i.e. those working in biotech and pharmaceutical companies) and non profit scientist working in basic research had surprisingly similar opinions. Though the percentage of industrial scientists that did not believe regulating pharmaceutical pricing was the answer was twice that of their non-profit counterparts, more than two thirds of both industrial and non-profit scientists believed regulation was necessary.
What this says about US scientists is that, regardless of who signs their paycheck, there is no an obvious bias on the topic of regulating pharmaceutical pricing practices. As one industrial scientist commented, “Government intervention is reaching a socialist level, but at the same time, a company should not be allowed to essentially have a monopoly on an orphan drug. What bothers me the most is that Valeant’s justification is [that they are] doing right by their shareholders, [which is] the crux of most of these issues. Wall Street gets richer, and the patients suffer.”.
Percepta, is a specialized firm that offers suppliers of research, applied and medical life science products and services market-specific insights and direction through primary market research and other professional services.


Leave a Reply