Approximately 570 diagnostic tests and testing systems have been approved by the FDA for exclusion from regulation under CLIA criteria. These tests are referred to as CLIA waived tests.
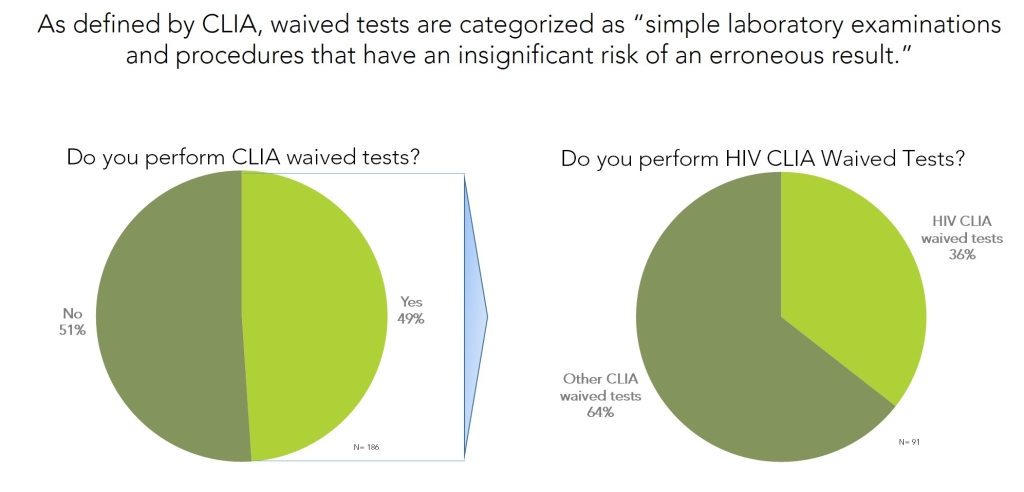
Roughly 4% of all CLIA waived tests in existence in the United States are HIV tests or test systems. Though all CLIA waived tests, including HIV tests, are designed to be easy to use they are not completely free from user error. As a result, it is relatively common for many of these CLIA waived tests to be performed in a point-of-care (POC) or other laboratory setting.
In CLIA waived lab settings HIV testing is among one of the more frequently performed tests. More than 1/3 of laboratories that perform CLIA waived tests include at least one form of HIV CLIA waived tests on their test menu.
Most Used HIV CLIA Waived Tests
Among the tests available the most prominently used includes those shown in the figure below:
Of the 25 HIV tests available, the top tests in use include the Bio-Rad Multispot HIV-1/HIV-2 Rapid Test, which detects HIV-1 and HIV-2 antibodies; the Abbott Architect i 2000 System, which detects HIV-1 and HIV-2 antigens and antibodies and the Hologic PROCLEIX ULTRIO Assay, which detects HIV-1 RNA. These three tests account for roughly 25% of the tests in use in reporting labs. However, each of these suppliers also provide other CLIA waived tests for detecting HIV, giving them a significant position of strength in the HIV CLIA waived test market.
Study Methodology
This study was performed by Percepta Associates Inc. as an online poll in February 2015 and included a total of 186 participants that performed CLIA waived tests in laboratories and POC settings in the United States.


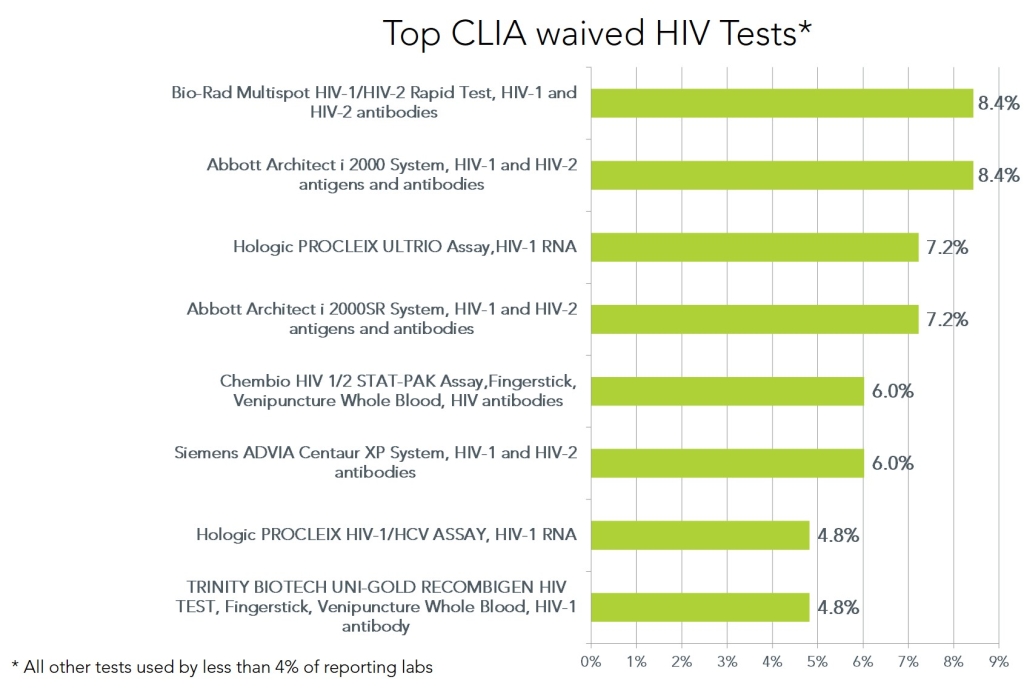
From the figure above, we can see that HIV-1/HIV-2 Rapid test and their antibodies are widely used in this field. so we hope all these methods and techniques can help scientists a lot in testing HIV.